What are flavonoids?
 Flavonoids, especially isolated quercetin glucosides, are polyphenolic compounds that offer an impressive array of health benefits. Many of the more than 5,000 flavonoid compounds have been found to have a low toxicity profile (1). Flavonoids occur naturally in numerous fruits and vegetables such as apples, onions, cherries and more.
Flavonoids, especially isolated quercetin glucosides, are polyphenolic compounds that offer an impressive array of health benefits. Many of the more than 5,000 flavonoid compounds have been found to have a low toxicity profile (1). Flavonoids occur naturally in numerous fruits and vegetables such as apples, onions, cherries and more.
The amount and bioavailability of flavonols in a particular plant species depends on many factors, including species, growing conditions, availability of light, ripeness, processing methods and means of preparation. Researchers have analyzed the chemical composition of the various flavonoids found in nature and divided them into the following classifications:
![]()
Numerous preclinical research studies have demonstrated that isoquercetin, a potent isolated quercetin glucoside, offers protection against inflammation, cardiovascular disease, allergies, neurological conditions and other chronic diseases. The evidence suggests that isoquercetin possesses powerful antioxidant and health enhancing properties. Although many plant species contain polyphenolic compounds, most dietary supplements contain the aglycone form of quercetin. This form of quercetin has a high molecular weight which severely limits bioavailability in the small intestine.
The flavonol isoquercetin
Quercetin and isoquercetin belong to a group of plant pigments called flavonols that give many fruits, flowers and vegetables their colour.
 Flavonols, including isolated isoquercetin, possess powerful antioxidant properties. Antioxidants are scavengers that search for free radicals in the body. Free radicals are harmful particles that move around the body striking cells and causing damage to DNA and cell membranes leading to cell death. Research indicates that antioxidants are able to neutralize free radicals. It is believed that flavonols produce an antihistamine and anti-inflammatory effect in the body. Flavonols may reduce allergy related symptoms such as swelling, watery eyes, hives and runny nose.
Flavonols, including isolated isoquercetin, possess powerful antioxidant properties. Antioxidants are scavengers that search for free radicals in the body. Free radicals are harmful particles that move around the body striking cells and causing damage to DNA and cell membranes leading to cell death. Research indicates that antioxidants are able to neutralize free radicals. It is believed that flavonols produce an antihistamine and anti-inflammatory effect in the body. Flavonols may reduce allergy related symptoms such as swelling, watery eyes, hives and runny nose.
Research studies in animals and humans suggest that the nutrients found in flavonols may help prevent plaque buildup, atherosclerosis and heart disease by lowering damage caused by LDL cholesterol. Research data also suggests that flavonols may relieve symptoms associated with high blood pressure, hypertension, interstitial cystitis, prostatitis and rheumatoid arthritis.
Supplementation with isolated natural isoquercetin derived from fruits and vegetables has also been associated with a lower risk of developing cancer, including lung, colon, breast, prostate, ovarian and endometrial tumors. Trace amounts of the yellow antioccident pigments concentrated in isoquercetin supplements can be found in various flowers, fruits and medicinal plants.
Sources of isoquercetin and quercetin
Isoquercetin is a polymorphic compound that can be isolated from plants such as rheum nobile, mango, noble rhubarb and sikkim rhubarb. Isoquercetin is the 3-O-glucoside of quercetin. Quercetin is the most abundant flavonoid molecule in the plant kingdom. The quercetin molecule can be found in vegetables, fruits, grains and in the leaves of plants. Quercetin and isoquercetin are widely used as ingredients in supplements, beverages and foods.
Chemical structure of isoquercetin
All flavonoids possess the same basic chemical structure. The molecule exhibits three rings with attached hydroxyl (OH) groups. Thousands of distinct flavonoids have been identified in nature based on a myriad of unique chemical substitutions and combinations.
Many plant based flavonoids feature a glycoside with a sugar molecule such as rhamnose, glucose or galactose attached to the C ring. Quercetin also serves as the aglycone, (minus the quercetin sugar molecule) of many flavonoids, including isoquercetin, rutin, hyperoside and quercetrin.
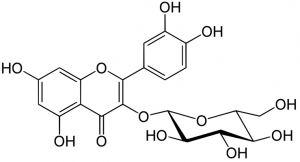 The molecular structure of these molecules is the same as quercetin, but one of the hydroxyl groups on the C ring has been replaced by a specific sugar molecule. Although quercetin provides a greater level of activity, molecular variations such as isoquercetin offer unique effects and activity levels.
The molecular structure of these molecules is the same as quercetin, but one of the hydroxyl groups on the C ring has been replaced by a specific sugar molecule. Although quercetin provides a greater level of activity, molecular variations such as isoquercetin offer unique effects and activity levels.
Quercetin’s antioxidant properties stem from its capacity to inhibit lipid peroxidation by chelating iron, blockading the xanthine oxidase enzyme and scavenging superoxide and hydroxyl, peroxy radicals. The antioxidative defense mechanism of Quercetin is activated by blocking the absorption of vitamin C14 and inhibiting protein damage. Quercetin also promotes the production and release of oxidative products produced by a respiratory burst related to phagocytes.
Naturally occurring quercetin contains only a small amount of aglycone. The compound consists of mostly glycosides. The glucoside chains are usually positioned at the 3 or 4 position of the pyrone. Most naturally occurring quercetin is converted into quercetin glucuronides as part of the digestive process.
As indicated previously, isoquercetin, a naturally occurring quercetin glucoside, is more bioavailable than the aglycone form of quercetin due to highly efficient hydrolysis and absorption of glucoside chains within the enterocytes.
The molecular formula for quercetin is C15H10O7, and the molar mass is 302.236 g/mol. Isoquercetin, with a molecular formula of C21H20O12, and a molar mass of 464.38 g/mol is sometimes referred to as quercetin-3-O-ß-D-glucoside, quercetin-3-O-glucoside, quercetin, hirsutrin or isoquercitrin. Isoquercitrin, or quercetin-3-monoglucoside, is functionally identical to isoquercetin, but isoquercetin has a pyranose ring and isoquercitrin has a furanose ring. The two names are used interchangeably in the literature.
Therapeutic effects of isoquercetin
With more than 10,000 published studies having already been completed, there’s little doubt that quercetin and isoquercetin have received more attention than any other flavonoid. The quercetin molecule has been shown to have cardiovascular, antioxidant, immune health and neurocognitive properties. The quercetin molecule has also demonstrated a diverse array of therapeutic applications, including enzyme activity, cellular signal transduction and gene expression. It is considered to be an archetypal flavonoid because of its unique ability to scavenge free radicals and donate electrons.
Isoquercetin may provide protection against brain ischemia and neuronal injuries caused by oxidation (2). In particular, the 3-methyl ether metabolite found in quercetin was able to inhibit lipid peroxidation, scavenge free radicals and protect against neuronal cell injury induced by oxidation and xanthine oxidase. Researchers have targeted the effectiveness of phyto chemicals with antioxidant potential against neurodegenerative diseases such as Alzheimer disease.
There are thousands of phytochemicals in the human diet, including the formidable antioxidant action of vitamin C. Quercetin, however, has proven to be an even stronger antioxidant and anticarcinogenic agent than vitamin C (3). Quercetin improves Cell viability and prevents the breakdown of neuronal cells. Oxidative stress and neurotoxicity are key components in the development of conditions such as Alzheimer’s disease, ischemic stroke and other chronic diseases.
Disease prevention
The glucosides in quercetin have been shown to provide protection against Alzheimer’s disease, cognitive decline and vascular dementia. Chronic quercetin administration was also able to reverse reserpine induced retention deficits in rats.4 Reserpine is a powerful neuroleptic drug administered to patients suffering from serious neurological conditions such as tardive dyskinesia.
It has been determined that compounds derived from quercetin 3-O-alpha-L-arabinopyranosyl, an isoquercetin compound derived from Eucommia ulmoide leaves, inhibited the formation of advanced glycation end products (5). Advanced glycation end products are formed by molecular mechanisms that cause diabetes complications.
In another study, the flavonoid glucoside isoquercetin was absorbed more rapidly and converted to glucuronidated quercetin because of the rapid hydrolysis of the sugar attached to the isoquercetin aglycone molecule (6). Isoquercetin has also been shown to provide protection against depression, especially when the flavonol compound is extracted from St. John’s wort. The structure of isoquercetin is surprisingly similar to the therapeutic compounds found in St. John’s wort (6). Animal studies suggest that the antidepressant qualities of St. John’s wort may stem from the regulation of cortisol and HPA-axis ACTH.
Bioavailability of quercetin and isoquercetin
The most common form of quercetin found in foods is quercetin-3-rutinoside. Quercetin-3-rutinoside is only one-fifth as bioavailable as quercetin-4′-glucoside (7). Researchers were able to convert Quercetin-3-rutinoside to quercetin-3-glucoside by cutting off a rhamnose molecule.
Splitting off the rhamnose molecule allowed the Quercetin-3-rutinoside to be just as bioavailable as quercetin-4′-glucoside. Quercetin glucoside is quickly absorbed in the small intestine of humans regardless of where the glucose moiety is positioned. Converting glycosides into glucosides represents one possible strategy for enhancing the bioavailability of the quercetin molecule in foods.
Unlike the vast majority of quercetin supplements, most of the quercetin found in foods is bound to a sugar molecule, a conjugate called glycoside. Although some foods, the apple for example, affix rutinose, yielding rutin to the quercetin molecule, plants such as the onion attach glucose to form quercetin-3-glucoside. The rutin yielding form of quercetin makes it difficult for the quercetin molecule to cross membrane barriers, but quercetin-3-glucoside, or isoquercetin, does not have that problem.
The transport mechanisms of isoquercetin
Researchers theorize that the glucose moiety sugar uses a transport mechanism similar to the mechanism used by glucose to cross the membrane barrier of the small intestine. Quercetin and rutin are unable to use this critical mechanism to promote rapid absorption (10). The rapid absorption of isoquercetin is strikingly similar to the means by which glucose crosses the intestinal wall. A research study revealed that quercetin required two to four hours to attain peak blood plasma levels (11). Rutin took six to eight hours to reach peak levels. Isoquercetin, on the other hand, required less than 40 minutes to attain peak concentrations.
Quercetin and isoquercetin are extremely effective α-glucosidase inhibitors. Researchers convincingly demonstrated that isoquercetin is more bioavailable than quercetin in diabetic KK -Ay mouse studies. The administration of isoquercetin led to an improvement in cholesterol, C-peptide, triglycerides and blood urea nitrogen levels in a month long clinical study.(8) Isoquercetin supplementation also promoted the function of pancreatic islets and improved glucose tolerance. Isoquercetin also helped regulate both blood glucose and lipid profiles.
In other research, isoquercetin has been identified as less lipophilic than quercetin aglycone. Direct comparisons of the bioavailability of isoquercetin, quercetin aglycone, and rutin have been made in rats. Rat bioavailability studies confirm that isoquercetin provides more small intestine bioavailability than quercetin aglycone and rutin (12). Rats that received isoquercetin attained peak blood plasma levels three to five times faster than rats that received quercetin aglycone or rutin.
Quercetin compounds are absorbed by brush border enterocytes in the wall of the small intestine. Quercetin glycosides featuring sugar moieties are absorbed in the lower parts of the intestine after going through a process known as deglycosylation. The glucosides are then absorbed into the epithelial cells by way of a sodium-dependent glucose transport mechanism known as SGLT1 (10).
Isoquercetin supplementation
Isoquercetin supplementation is being touted as a way to combat aging and age related disease. The goal is nothing less than to enhance cell energy metabolism and cell energy management.
Scavenges free radicals
Promotes DNA integrity
Supports cellular regulation and cardiovascular health
Not only is isoquercetin far more bioavailable than quercetin, it exerts a stronger physiological response in the human body. The benefits of isolated isoquercetin supplementation are revolutionizing the way antiaging researchers and health practitioners think about disease prevention and life span longevity. Rapidly absorbed natural isoquercetin has superceded quercetin and resveratrol in the quest to reduce the effects of oxidative stress while improving metabolic homeostasis and mitochondrial function to achieve health and longevity (13).
Is it really possible to control mitochondrial biogenesis and function? Researchers have reason to believe that the time has come to employ interventions that possess the power to improve overall health and extend life.
Isoquercetin supplements have taken the antioxidant revolution to the next level of natural health care management. Isoquercetin supplementation holds the promise of enhancing the effects of even moderate exercise, fighting inflammation and curing chronic disease.
References:
(1)Evaluating the bioavailability of isoquercetin.
J Appleton Natural Medicine Journal 2010 January;Vol. 2 Issue 1(2)Neuroprotective effects of antioxidative flavonoids, quercetin, (+)-dihydroquercetin and quercetin 3-methyl ether, isolated from Opuntia ficus-indica var. saboten.
H Dok-Go et al Brain Res 2003 Mar 7;965(1-2):130-6.(3)Protective effects of Quercetin and vitamin C against oxidative stress-induced neurodegeneration.
J Agric Food Chem 2004;52 (25), pp 7514–7517.DOI: 10.1021/jf049243r.(4)Reversal of reserpine-induced orofacial dyskinesia and cognitive dysfunction by quercetin.
PS Naidu et al PMID 2004 Feb;70(2):59-67.(5)Flavonol glycosides from the leaves of Eucommia ulmoides O. with glycation inhibitory activity.
HY Kim et al PMID 2004 Aug;93(2-3):227-30.DOI: 10.1016/j.jep.2004.03.047.(6)Difference in absorption of the two structurally similar flavonoid glycosides, hyperoside and isoquercitrin, in rats.
Q Chang et al PMID 2005 Apr;59(3):549-55.DOI: 10.1016/j.ejpb.2004.10.004.(7)Bioavailabilities of quercetin-3-glucoside and quercetin-4′-glucoside do not differ in humans.
MR Olthof et al 2000 May;130(5):1200-3.(8)Antidiabetic activity of isoquercetin in diabetic KK -Ay mice.
R Zhang et al 2011 Dec 2;8:85. doi: 10.1186/1743-7075-8-85.(9)Relative bioavailability of the antioxidant flavonoid quercetin from various foods in man.
PC Hollman et al FEBS Lett 1997;418(1-2):152-156.(10)Intestinal transport of quercetin glycosides in rats involves both deglycosylation and interaction with the hexose transport pathway.
JM Gee et al J Nutr 2000;130(11):2765-2771.(11)Bioavailabilities of quercetin-3-glucoside and quercetin-4′-glucoside do not differ in humans.
MR Olthof et al J Nutr 2000;130(5):1200-1203.(12)Bioavailability of rutin and quercetin in rats.
C Manach et al FEBS Lett 1997;409(1):12-16.(13)Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha.
M Lagouge PMID 2006 Dec 15;127(6):1109-22. Epub 2006 Nov 16.DOI: 10.1016/j.cell.2006.11.013.